High biochemical remission rates in patients with primary biliary cholangitis treated with “triple” anticholestatic therapy
October 2025
Abstract
Background:
Treatment goals in primary biliary cholangitis (PBC) are increasingly aspirational, aiming for normal serum liver tests. One of the add-on therapies to ursodeoxycholic acid (UDCA) is with the approved farnesoid X receptor (FXR) agonist obeticholic acid (OCA), alongside off-label use of fibrates (peroxisome proliferator-activated receptor [PPARs]). We report our experience of synergistic FXR-PPAR-UDCA combination therapy in PBC.
Methods:
A review of patients with PBC seen between July 2022 and July 2023 was performed across the autoimmune liver disease programme at the Toronto Centre for Liver Disease. Univariate and multivariate analyses were performed.
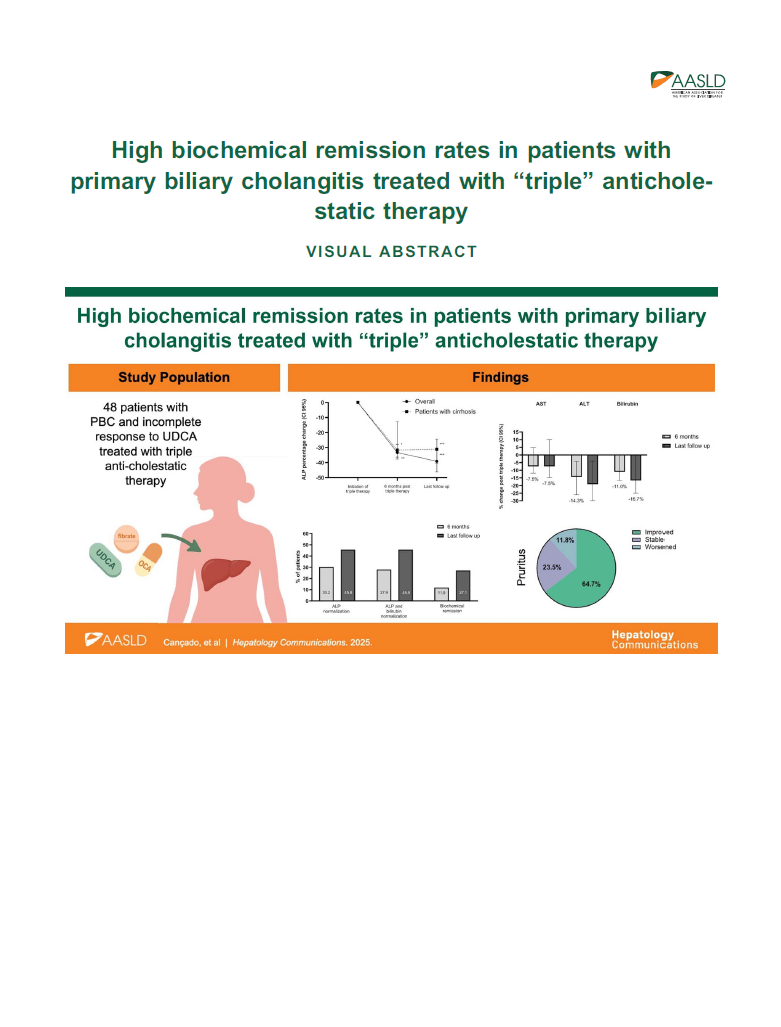
Results:
Four hundred seventy patients with PBC were seen, of which 71% were treated with UDCA only, 7% UDCA-OCA, 11.3% UDCA-fibrates, and 10.6% UDCA-OCA-fibrates. Among 50 patients on triple therapy, 82% had OCA as the first add-on therapy. Most patients (92%) received bezafibrate, while 8% had fenofibrate. Forty-eight patients were included in the final analysis. The mean follow-up time after triple therapy was 17.4 months. Triple therapy demonstrated median ALP reductions after 6 months of 33.3% (95% CI: 27.9%–37.6%) and 39.1% (95% CI: 30.7%–46.2%) at the last follow up; 30.2% of the patients had a normal serum ALP at 6 months, while 11.9% had normal ALP, AST, ALT, and bilirubin. Subgroup analysis of 28 patients followed for at least 12 months showed a 44.7% (95% CI: 33.3%–50.9%) median reduction in ALP. Liver stiffness remained relatively stable throughout the follow-up. Out of 34 patients with self-reported pruritus before triple therapy, 64.7% reported improvement, 11.8% worsened, and 23.5% had no change in itching intensity. On multivariable analysis, only older age at diagnosis (OR=1.12; 95% CI: 1.02–1.22) positively impacted ALP normalization.
Conclusions:
Our data confirm that FXR-PPAR-UDCA triple therapy significantly improves ALP with normalization for 30% of patients with PBC at 6 months.
Hepatology Communications. © 2025 Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Note: Obeticholic acid, marketed under the brand name Ocaliva® for the treatment of primary biliary cholangitis (PBC), was voluntarily withdrawn from the US market by Intercept Pharmaceuticals following a request from the US Food and Drug Administration (FDA) on 11/14/2025